铵盐也是二次污染气溶胶的最重要的组分之一。铵盐主要是以(NH 4)2 SO 4和NH 4 NO 3的形式,存在于对流层中[28]。图60-5显示 了 北 京2001—2003年TSP和PM 2.5中铵盐浓度的季节变化,这里NH 4+代表(NH 4)2 SO 4和NH 4 NO 3。冬季和夏季铵盐的浓度高于秋季、春季和沙尘暴期间。冬季铵盐的浓度在TSP和PM 2.5中分别为22.9和12.8μg·m-3;夏季则为17.1和7.6μg·m-3,均高于秋季(9.1和6.6μg·m-3)、春季(5.5和5.3μg·m-3)和沙尘暴期间(1.6和1.5μg·m-3)。此外,铵盐在PM 2.5和TSP中的质量浓度比值(PM 2.5/TSP)约为60%,说明铵盐较多存在于细粒子中。图60-5也显示了TSP和PM 2.5中矿物气溶胶与铵盐的季节变化。铵盐的季节变化与矿物气溶胶有些相似之处,它们在TSP和PM 2.5中的相关系数分别为0.62和0.87(P<0.01),说明矿物气溶胶和铵盐之间存在着正相关性。2003年春季和秋季,铵盐的浓度直接随着矿物气溶胶浓度的增加而增加,表现出很好的正相关性。气态NH 3与大气中的酸性气体H 2 SO 4和HNO3或矿物气溶胶表面的H 2 SO 4/HNO 3发生反应,随后冷凝在矿物气溶胶表面,形成(NH 4)2 SO 4和NH 4 NO 3。铵盐的形成不仅和它的前体物NH 3、SO 2和NO 2的浓度以及气象因素有关,还与矿物气溶胶的浓度有关。2002年夏季铵盐的浓度较高,且随着矿物气溶胶浓度的增加而增加,表现出好的正相关性。这是因为夏季NH 3和NO 2浓度较高,相对湿度较高,温度也较高,且风速低,有利于铵盐的形成。但是,温度越高,NH 4 NO 3越易分解,因此夏季铵盐的浓度低于冬季。2001年冬季,尽管大气中NH 3表现出较低的浓度[29,30],但是铵盐的浓度仍然较高,且随矿物气溶胶浓度的增加而增加。这是因为有较高浓度的SO 2、NO 2和矿物气溶胶,再加上较低的温度,有助于铵盐的形成。2002年沙尘暴期间,由于它的前体物NH 3、SO 2和NO 2浓度最低,再加上非常不利的气象条件、最强的风速、最低的相对湿度,使得铵盐不易形成,故铵盐的浓度与其他季节相比是最低的,在TSP和PM 2.5中分别为1.6和1.5μg·m-3。即便在沙尘暴期间,PM 2.5中铵盐占TSP中铵盐的95%,说明铵盐绝大部分存在于细粒子中。与硝酸盐非常类似,铵盐在沙尘暴期间明显地随矿物气溶胶浓度的增加而减少;沙尘暴高峰过后,铵盐又随着矿物气溶胶浓度的减少而减少,说明铵盐主要来自当地污染源。
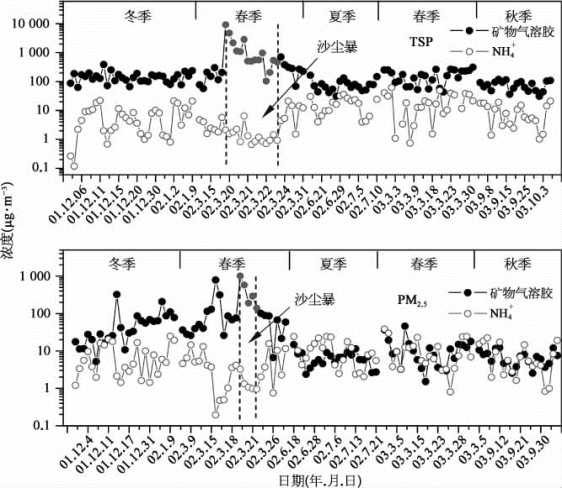
图60-5 TSP和PM 2.5中矿物气溶胶浓度与铵盐浓度的季节变化(彩图见下载文件包,网址见14页脚注)
参考文献
[1] D'Almeida G A,Koepke P,Shettle E P.Atmospheric aerosols:Global climatology and radiative characteristics.Hampton,Virginia:A.Deepak Publishing,1991:55-59.
[2] Jonas P,Charlson R,Rodhe H.Aerosols//Houghton J T,et al.Climate change 1994.Cambridge:Cambridge University Press,1995:92-128.
[3] Zhang X Y,Arimoto R,An Z S.Dust emission from Chinese desert sources linked to variations in atmospheric circulation.Journal of Geophysical Research,1997,102:28041-28047.
[4] Andreae M O.Climate effects of changing atmospheric aerosol levels//Henderson-Sellers A.World survey of climatology:Future climates of the world.New York:Elsevier,1995:341-392.
[5] Duce R A.Sources,distributions and fluxes of mineral aerosols and their relationship to climate//Heintzenberg J.Aerosol forcing of climate.New York:Wiley,1995:43-72.
[6] Zhuang G S,Yi Z,Duce R A,et al.Chemistry of iron in marine aerosols.Global Biogeochemical Cycles,1992a,12:171-179.
[7] Zhuang G S,Yi Z,Duce R A,et al.Link between iron and sulfur suggested by the detection of Fe(Ⅱ)in remote marine aerosols.Nature,1992b,355:537-539.
[8] Zhuang G S,Guo J H,Yuan H,et al.Coupling and feedback between iron and sulfur in air-sea exchange.Chinese Science Bulletin,2003,48(8):1080-1086.
[9] Bian H S,Zender C S.Mineral dust and global tropospheric chemistry:Relative roles of photolysis and heterogeneous uptake.Journal of Geophysical Research:Atmospheres,2003,108(D21):4672-4687.
[10] Underwood G M,Li P,Al-Abadleh H,et al.A Knudsen cell study of the heterogeneous reactivity of nitric acid on oxide and mineral dust particles.Journal of Physical Chemistry A,2001,105(27):6609-6620.
[11] Song C H,Carmichael G R.A three-dimensional modeling investigation of the evolution processes of dust and sea-salt particles in East Asia.Journal of Geophysical Research:Atmosphere,2001,106(D16):18131-18154.
[12] Zhang D,Iwasaka Y.Nitrate and sulfate in individual Asian dust-storm particles in Beijing,China in spring of 1995 and 1996.Atmospheric Environment,1999,33:3213-3223.
[13] Yaacov M,Judith G.Heterogeneous reactions of minerals with sulfur and nitrogen oxides.Journal of Aerosol Science,1989,20(3):303-311.
[14] Okada K,Naruse H,Tanaka T,et al.X-ray spectrometry of individual Asian dust-storm particles over the Japanese islands and the North Pacific Ocean.Atmospheric Environment,1990,24:1369-1378.(https://www.xing528.com)
[15] Dentener F J,Carmichael G R,Zhang Y.Role of mineral aerosol as a reactive surface in the global troposphere.Journal of Geophysical Research:Atmospheres,1996,101(D17):22869-22889.
[16] Chen L,Carmichael G R,Hong M S,et al.Influence of continental outflow events on the aerosol composition at Cheju Island,South Korea.Journal of Geophysical Research:Atmospheres,1997,102(D23):28551-28574.
[17] Taylor S R,Mc Lennan S M.The continental crust:Its composition and evolution.New York,Oxford:Blackwells,1985.
[18] Zhang X Y,An Z S,Liu D S,et al.Study on three dust storm in China.Chinese Science Bulletin,1992,39(11):940-945.
[19] 韩力慧,庄国顺,孙业乐,等.北京大气颗粒物污染的本地源与外来源.中国科学B辑:化学,2005,35(3):237-246.
[20] Han L H,Zhuang G S,Sun Y L,et al.Local and nonlocal sources of airborne particulate pollution at Beijing.Science in China Series B:Chemistry,2005,48(3):247-258.
[21] Nishikawa M,Kanamori S.Chemical composition of kosa aerosol(yellow sand dust)collected in Japan.Analytical Science,1991,7:1127-1130.
[22] Nishikawa M,Kanamori S,Kanamori N.Kosa aerosol as eolian carrier of anthropogenic material.Science of the Total Environment,1991,107:13-27.
[23] 肖辉,Carmichael G R,Zhang Y.东亚地区沙尘气溶胶影响硫酸盐形成的模式评估.大气科学,1998,22(3):343-353.
[24] Zhang X Y,Zhuang G S,Chen J M,et al.Heterogeneous reactions of sulfur dioxide on typical mineral particles.Journal of Physical Chemistry B,2006,110:12588-12596.
[25] Yuan H,Rahn K A,Zhuang G S.Graphical techniques for interpreting the composition of individual aerosol particles.Atmospheric Environment,2004,38:6845-6854.
[26] Yuan H,Zhuang G S,Rahn K A,et al.Composition and mixing of individual particles in dust and nondust conditions of North China,Spring 2002.Journal of Geophysical Research,2006,111:D20208.doi:10.1029/2005JD006478.
[27] Iwasaka Y,Shi G Y,Shen Z,et al.Nature of atmospheric aerosols over the desert areas in the Asia Continent:Chemical state and number concentration of particles measured at Dunhuang,China.Water,Air and Soil Pollution:Focus,2003,3:129-145.
[28] Wang Y,Zhuang G S,Tang A H,et al.The ion chemistry of PM 2.5 aerosol in Beijing.Atmospheric Environment,2005,39(21):3771-3784.
[29] 孙庆瑞,王美蓉.中国氨的排放量和时空分布.大气科学,1997,21(5):590.
[30] 彭应登,杨名珍,申立贤.北京氨源排放及其对二次粒子生成的影响.环境科学,2000,21:101-103.
免责声明:以上内容源自网络,版权归原作者所有,如有侵犯您的原创版权请告知,我们将尽快删除相关内容。




