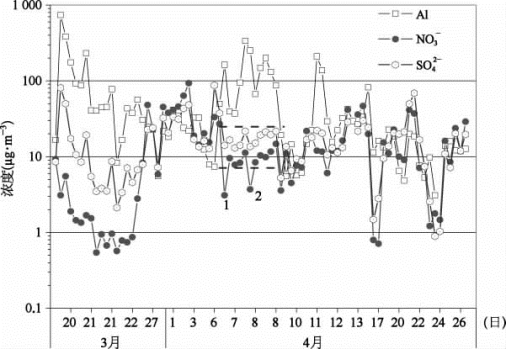
图54-7 2002年春季3、4月份Al、硫酸盐和硝酸盐的时间变化图(彩图见下载文件包,网址见14页脚注)

图54-8 2002年春季3月19日—4月27日硫酸盐与Al、硝酸盐与Al关系图(彩图见下载文件包,网址见14页脚注)
长距离传输过程中,沙尘颗粒物表面硫酸盐和硝酸盐的形成已经被实验室模拟、模式计算以及单颗粒物分析所证实[10,11,28 30]。先前的研究也发现,硫酸盐和硝酸盐可能存在矿物源[13,31]。图54-7显示了2002年春季3月19日—4月27日元素Al、硫酸盐以及硝酸盐的浓度变化。很明显,DSⅠ和DSⅡ中的硫酸盐和硝酸盐有明显不同。DSⅠ中的Al、硫酸盐和硝酸盐有相同的变化趋势;而在DSⅡ中,当Al的浓度变化时,硫酸盐和硝酸盐的浓度基本上没有什么变化。如图54-8所示,DSⅠ中的硫酸盐和Al有很好的相关性(相关系数为0.98),硝酸盐和Al也有较好的相关性(相关系数为0.71),说明硫酸盐和硝酸盐部分来自地壳源。如果所有的S元素均以硫酸盐形式存在的话,那么[SO 24-]/S比值应该为3.0。DSⅠ中的[SO 24-]/S比值为1.79{[SO 24-],对S的线形回归方程为[SO24-]=-0.40+1.7S(r=0.93)},说明有大约一半的S以不可溶性形态存在,而这个部分极有可能来自源区的土壤尘,或者其与沿途扬尘的混合。DSⅠ中[SO 24-]/Al和[NO 3-]/Al比值分别为0.12和0.016,显著高于中国戈壁滩以及黄土高原表层土壤中的值([SO 24-]/Al为0.002,[NO 3-]/Al<0.002[13])。其硫酸盐和硝酸盐的质量百分比,分别为0.66%和0.095%,也显著高于上面所述地区表层土壤中硫酸盐和硝酸盐的含量(硫酸盐为0.01%,硝酸盐<0.01%[13])。这些结果清楚地表明,除了沙尘源,DSⅠ中的硫酸盐和硝酸盐必定还有其他来源。例如可能来自沙尘颗粒表面SO 2、NO x以及H 2 SO 4和HNO3的异相反应或者长距离传输过程中沙尘颗粒物的表面吸附。DSⅡ中的硫酸盐和硝酸盐与Al没有相关性。当Al从37μg·m-3(图54-8点c)变化到336μg·m-3(图54-8点d)时,硫酸盐和硝酸盐的变化分别为13.1~24.2和7.1~14.8μg·m-3,均在2倍左右。DSⅡ沿着相对清洁的路径进行传输,而该地区SO 2和NO x的排放量相对较少,因此沙尘颗粒表面的SO2和NO x的反应相对较少。另外DSⅡ的相对强度弱于DSⅠ,沙尘来之前冷空气团对污染物的清除能力必定也弱于DSⅠ[32],这样,DSⅡ的入侵气团也会更多地与北京本地源污染物相混合。因此DSⅡ中硫酸盐和硝酸盐的浓度与非沙尘暴期间的浓度比较接近,而且没有什么大的变化。如图54-8所示,DSⅡ中硫酸盐的浓度为18μg·m-3,非常接近非沙尘暴期间的浓度19μg·m-3,硝酸盐的浓度9μg·m-3则大大低于非沙尘暴期间的浓度20μg·m-3。DSⅡ中低浓度的硝酸盐归因于粗颗粒态硝酸盐的沉降。图54-7和图54-8(b)中的1和2点两个样品的硝酸盐浓度最低,这2个样品均采集于夜间,而夜间HNO3与碳酸盐的反应更为有效,由此生成的粗颗粒态硝酸盐由于其重力作用而更容易沉降,因此这2个夜间样品的硝酸盐浓度非常低。
矿物气溶胶颗粒物表面SO2和H 2 SO4的异相反应,强烈地取决于湿度,其转化速率随着湿度的增加而增加[33,34]。在距离源区较远以及在夜间的边界层,湿度相对较大,SO2容易被沙尘颗粒表面所吸收,进而被O3、H 2 O2和OH自由基氧化。如前面所提,DSⅠ经过许多高硫排放的煤矿区和“污染”城市[35]。很明显,高浓度的SO 2加上沙尘颗粒表面固有的碱性,有利于SO 2的吸收和反应。NO x和HNO 3在沙尘颗粒表面反应之后生成的一小薄层,也得到证实[36,37]。HNO 3可以同沙尘颗粒表面的液态碳酸盐反应,生成粗颗粒态的硝酸盐,成为NO x沉降的一个重要过程。其反应机理为[38]:
![]()
DSⅠ和DSⅡ中硫酸盐和硝酸盐的显著差异,表明传输路径对化学转化过程,进而对沙尘暴长距离传输过程中的气溶胶组成,具有重要影响。沙尘暴可以作为硫酸盐和硝酸盐形成的表面载体。硫酸盐与矿物颗粒的相关性,说明有部分硫酸盐气溶胶,来自一次排放的、由古海洋源沙漠产生的沙尘气溶胶。这部分硫酸盐对全球产生降温效应[28]。而硝酸盐与矿物颗粒相关,则会增加其传输距离;而传输到北太平洋地区的硝酸盐,又会在海洋边界层同海盐气溶胶反应,产生Cl原子[4],进而对全球生物地球化学循环产生深远影响。
综上所述,2002年春季北京遭受了一系列沙尘暴。根据后向轨迹和PM 10浓度判断出来的传输路径,可以将其分为2类——DSⅠ和DSⅡ。DSⅠ主要来源于内蒙古中西部的沙漠以及黄土高原,而DSⅡ则主要来自蒙古戈壁滩以及内蒙古北部的沙地。西路传输路径可以被看作是“污染”路径,而西北偏北传输路径则为相对“清洁”路径。沙尘暴不仅输送了大量的矿物元素,而且携带了相当数量的污染元素。源区和传输路径是影响沙尘暴化学组成最重要的2个因素。由于不同源区Ca的含量有明显差异,因此Ca/Al可被用作元素示踪体系,以判断沙尘暴的来源。沿“污染”路径传输的DSⅠ,要比沿“清洁”路径的DSⅡ,携带了更多的污染元素。这些污染元素或者来自土壤尘(如Zn),或者来自沙尘与沿途污染气溶胶的混合(如DSⅠ的中As和Pb),或者来自沿途以及北京本地的“污染”扬尘(如DSⅡ中的As和Pb)或沙尘颗粒表面的反应(如S)。沙尘矿物气溶胶可以为硫酸盐和硝酸盐的形成提供反应界面,进而对全球生物地球化学循环产生深远影响。
参考文献
[1] Hee J I,Soon U P.A simulation of long-range transport of Yellow Sand observed in April 1998 in Korea.Atmospheric Environment,2002,36(26):4173-4187.
[2] Duce R A,Unni C K,Ray B J.Long range atmospheric transport of soil dust from Asia to the Tropical North Pacific:Temporal variability.Science,1980,209:1522-1524.
[3] Uematsu M,Duce R A,Prospero J M,et al.Transport of mineral aerosol from Asia over the North Pacific Ocean.Journal of Geophysical Research,1983,88:5343-5352.
[4] Uematsu M,Yoshikawa A,Muraki et al.Transport of mineral and anthropogenic aerosols during a Kosa event over East Asia.Journal of Geophysical Research-Atmospheres,2002,107:D7-D8.
[5] Chung Y S,Kim H S,Dulam J,et al.On heavy dustfall with explosive sandstorms in Chongwon-Chongju,Korea in 2002.Atmospheric Environment,2003,37:3425-3433.
[6] Sun Y,Zhuang G S,Yuan H,et al.Characteristics and sources of 2002 super dust storm in Beijing.Chinese Science Bulletin,2004,49(7):698-705.
[7] Husar R B,Tratt D M,Schichtel B A,et al.Asian dust events of April 1998.Journal of Geophysical Research-Atmospheres,2001,106(D16):18317-18330.
[8] Liang Q,Jaegle L,Jaffe D A,et al.Long-range transport of Asian pollution to the Northeast Pacific:Seasonal variations and transport pathways of carbon monoxide.Journal of Geophysical Research-Atmospheres,2004,109(D23).
[9] Huebert B J,Bates T,Russell P B,et al.An overview of ACE-Asia:Strategies for quantifying the relationships between Asian aerosols and their climatic impacts.Journal of Geophysical Research-Atmospheres,2003,108(D23).
[10] Iwasaka Y,Yamato M,Imasu R A O.Transport of Asian dust(KOSA)particles;importance of weak KOSA events on the geochemical cycle of soil particles.Tellus,1988,40B:494-503.
[11] Okada K,Naruse H,Tanaka T,et al.X-ray spectrometry of individual Asian dust-storm particles over the Japanese islands and the North Pacific Ocean.Atmospheric Environment,1990,24:1369-1378.
[12] Zhou M,Okada K,Qian F W,et al.Characteristics of dust-storm particles and their long-range transport from China to Japan—Case studies in April 1993.Atmospheric Research,1993,40(1):19-31.
[13] Nishikawa M,Kanamori S,Kanamori N,et al.Kosa aerosol as aeolian carrier of anthropogenic material.Science of The Total Environment,1991,107:13-27.
[14] Gao Y,Arimoto R,Duce R A,et al.Input of atmospheric trace elements and mineral matter to the Yellow Sea during the spring of a low-dust year.Journal of Geophysical Research[Atmospheres],1992,97(D4):3767-3777.
[15] Arimoto R,Duce R A,Savoie D L,et al.Relationships among aerosol constituents from Asia and the North Pacific Ocean during PEM-West A.Journal of Geophysical Research,1996,101(D1):2011-2023.(https://www.xing528.com)
[16] Zhuang G S,Yi Z,Duce R A,et al.Chemistry of iron in marine aerosols.Global Biogeochemical Cycles,1992,6(2):161-173.
[17] Zhuang G S,Guo J H,Yuan H,et al.The compositions,sources,and size distribution of the dust storm from China in spring of 2000 and its impact on the global environment.Chinese Science Bulletin,2001,46(11):895-901.
[18] Zhang X Y,Arimoto R,An Z S.Dust emission from Chinese desert sources linked to variations in atmospheric circulation.Journal of Geophysical Research,1997,23:28041-28047.
[19] Gao Y,Arimoto R,Zhou M Y,et al.Relationships between the dust concentrations over eastern Asia and the remote North Pacific.Journal of Geophysical Research[Atmospheres],1992,97(D9):9867-9872.
[20] Sun J M,Zhang M Y,Liu T S.Spatial and temporal characteristics of dust storms in China and its surrounding regions,1960-1999:Relations to source area and climate.Journal of Geophysical Research-Atmospheres,2001,106(D10):10325-10333.
[21] Xuan J,Sokolik I N.Characterization of sources and emission rates of mineral dust in Northern China.Atmospheric Environment,2002,36(31):4863-4876.
[22] Zhang X Y,Gong S L,Shen Z X,et al.Characterization of soil dust aerosol in China and its transport and distribution during 2001 ACE-Asia:1.Network observations.Journal of Geophysical Research-Atmospheres,2003,108(D9).
[23] Draxler R R,Hess G D.An overview of the Hysplit-4 modeling system for trajectories,dispersion,and deposition.Australia Meteorology Magazine,1998,47:295-308.
[24] Taylor S R,Mc Lennan S M.The geochemical evolution of the continental crust.Review of Geophysics,1995,33:241-265.
[25] Mason B,Moore C B.Principles of Geochemistry:4th ed.New York:Wiley,1982:45-47.
[26] Zhang X Y,Zhang G Y,Zhu G H,et al.Elemental tracers for Chinese source dust.Science in China(Ser D),1996,39(5):512-521.
[27] Makra L,Borbely-Kiss I,Koltay E,et al.Enrichment of desert soil elements in Taklimakan dust aerosol.Nuclear Instruments and Methods in Physics Research B,2002,189:214-220.
[28] Dentener F J,Carmichael G R,Zhang Y,et al.Role of mineral aerosol as a reactive surface in the global troposphere.Journal of Geophysical Research-Atmospheres,1996,101(D17):22869-22889.
[29] Song C H,Carmichael G R.A three-dimensional modeling investigation of the evolution processes of dust and sea-salt particles in East Asia.Journal of Geophysical Research-Atmospheres 2001,106(D16):18131-18154.
[30] Underwood G M,Li P,Al-Abadleh H,et al.A Knudsen cell study of the heterogeneous reactivity of nitric acid on oxide and mineral dust particles.Journal of Physical Chemistry A,2001,105(27):6609-6620.
[31] Prospero J M,Savoie D L.Effect of continental sources of nitrate concentrations over the Pacific Ocean.Nature,1989,33:687-689.
[32] Guo J,Rahn K A,Zhuang G S.A mechanism for the increase of pollution elements in dust storms in Beijing.Atmospheric Environment,2004,38(6):855-862.
[33] Haury G,Jordan S,Hofmann C.Experimental investigations of the aerosol-catalyzed oxidation of SO 2 under atmospheric conditions.Atmospheric Environment,1978,12:281-287.
[34] Dlugi R,Jordan S,Lindemann E.The heterogeneous formation of sulfate aerosols in the atmosphere.Journal of Aerosol Science,1981,12:185-197.
[35] Akimoto H,Narita H.Distribution of SO 2,NO x and CO 2 emissions from fuel combustion and industrial activities in Asia with 1°×1° resolution.Atmospheric Environment,1994,28:213-225.
[36] Mamane Y,Gottlieb J.Heterogeneous reaction of nitrogen oxides on sea salt and mineral particles—A single particle approach.Journal of Aerosol Science,1990,21(Suppl.1):S225-S228.
[37] Wu P M,Okada K.Nature of coarse nitrate particles in the atmosphere—A single particle approach.Atmospheric Environment,1994,28:2053-2060.
[38] Mamane Y,Gottlieb J.Nitrate formation on sea-salt and mineral particles—A single approach.Atmospheric Environment,1992,26A:1763-1769.
免责声明:以上内容源自网络,版权归原作者所有,如有侵犯您的原创版权请告知,我们将尽快删除相关内容。




