紫外吸收光谱中常用的溶剂有己烷、庚烷、环己烷、二氧杂己烷、水、乙醇等。应当注意的是,有些溶剂,特别是极性溶剂,对溶质吸收峰的波长、强度及形状可能产生影响。这是因为溶剂和溶质间常形成氢键,或溶剂的偶极使溶质的极性增强,引起π→π*及n→π*吸收带的迁移。例如,异丙叉丙酮的溶剂效应见表5-6。
表5-6 异丙叉丙酮的溶剂效应
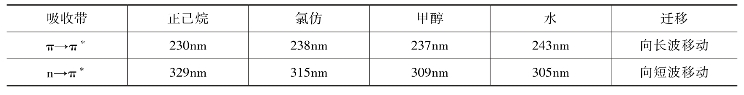
溶剂除了对吸收波长有影响外,还影响吸收强度和精细结构。例如B吸收带的精细结构在非极性溶剂中较清楚,但在非极性溶剂中则较弱,有时消失而出现一个宽峰。如苯酚的B吸收带在非极性溶剂庚烷中清晰可见,而在极性溶剂乙醇中则完全消失而呈现一宽峰。因此,在溶解度允许的范围内,应选择极性较小的溶剂。
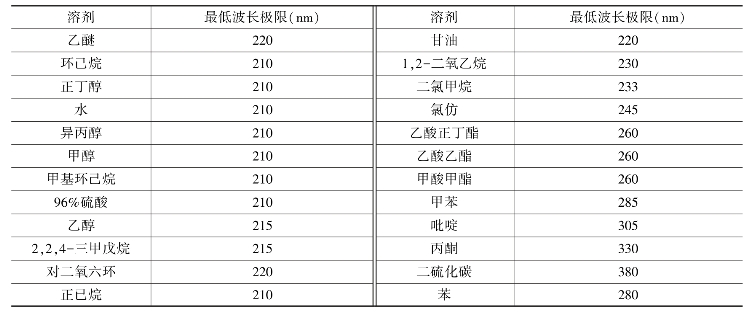
此外,溶剂本身有一定的吸收带,如果和溶质的吸收带有重叠,将妨碍溶质吸收带的观察。表5-7是紫外吸收光谱分析中常用溶剂的最低波长极限,低于此波长时,溶剂的吸收不可忽略。(https://www.xing528.com)
表5-7 溶剂的最低使用波长极限
紫外光谱对溶剂的要求用英文描述如下:
Ultraviolet or visible spectra are recorded in very dilute solutions.The solvent chosen for preparing solutions of the sample must be capable of dissolving the substance and transmitting in the wavelength region under study.Hexane,ethanol and cyclohexane are the most common solvents used as they are cheap and transparent down to about 210 nm.A suitable quantity of the substance is dissolved in an appropriate solvent.The concentration of the solution and the size of the cell are so adjusted as to give absorbance between 0.3 and 1.0——a value convenient for measurement.A portion of the solution is transferred into the silica cell and placed after the monochromator to avoid fluorescence or possible decomposition due to the absorption of other high energy wavelengths of unresolved radiation (the IR absorption cell is placed before the monochromators).A similar cell containing only pure solvent is also placed along with the sample cell and both are mounted in a special holder in such a way that two equal beams of UV light are passed –one through the sample cell and the other through the solvent cell.
免责声明:以上内容源自网络,版权归原作者所有,如有侵犯您的原创版权请告知,我们将尽快删除相关内容。




