赵敏寿 赵奇金 唐定骧
(中国科学院长春应用化学研究所)
摘 要:用线性扫描伏-安法研究了等摩尔KCl·NaCl熔体中钕和钇离子在锑、铋、铝、镓、锡、锌、铅、铟八种液体阴极和固体钼阴极上析出电位,求出了析出电位差Δφ。产生Δφ的原因是稀土在液体阴极中的活度比在固体阴极上低,使析出电位向正移,同时也由于稀土与液体阴极合金化产生去极化作用的结果。稀土金属和液体阴极材料的电负性差越大,Δφ也越大,还研究了稀土氯化物浓度和熔体温度对稀土离子析出电位和Δφ的影响,给出了相应的关系式。
Investigation of Deposition Potential of Neodymium and Yttrium Ions on Liquid Cathodes in Molten Chloride
Zhao Minshou Zhao Qijin Tang Dingxiang
(Changchun Institute of Applied Chemistry,Academia Sinica)
Abstract:Deposition potentials of neodymium and yttrium ions on liquid Sb,Bi,Al,Ga,Sn,Zn,Pb and In cathodes and Solid Mo cathode in equimolar KCl·NaCl have been studied using technique of potential sweep voltammetry.The deposition potential differences between liquid cathodes and Mo cathodeΔφhave been calculated.These differences are not only due to decreased activity of neodynium and yttrium in liquid cathodes,shifting deposition potentials to oppositive direction but also the depolarization effect caused by alloying neodymium and yttrium with liquid cathode.The greater electronegativity differences between rare earth metals and liquid cathode materials,the greater isΔφ.Effects of NdCl3 and YCl3 concentrations and temperature of the melt on deposition potentials of neodymium and yttrium ions andΔφ have been studied as well.Corresponding equations are given.
一、前言
随着液体阴极制取稀土中间合金工作的发展,液体阴极化过程研究已有一些报道[1-3]。钕和铒在铅阴极上的极化过程研究[4],镧系和钇十五种稀土离子在液体铝阴极上的析出电化序工作,都取得了可喜的结果[5]。
本工作研究碱金属氯化物熔体中,钕和钇在液体阴极上的极化过程,并测定钕和钇离子的析出电位。探讨钕和钇在液体阴极上析出电位较在惰性阴极上明显偏正的原因,是稀土熔盐化学的基础研究课题之一,也为制取稀土中间合金寻找适宜的阴极材料和选择工艺参数提供依据。
二、实验程序和装置
电解槽结构和实验装置见文献[5]。实验在氩气保护下进行,用上升线性波电位扫描,扫描范围0~2.5伏,周期10分钟。用银一氯化银参比电极。固体钼电极S固=0.225厘米2,最大电流密度dmax=4.55安/厘米2,液体阴极面积S液=1.31厘米2,最大电流密度dmax=0.76安/厘米2。
三、实验结果和讨论
(一)钕和钇在钼和液体阴极上的析出电位
800℃下等摩尔KCl·NaCl熔体中,钕和钇在固体钼及液体阴极上析出电位和两者之间的电位差Δφ,列于表1。
由表1得知:钕和钇离子在各种液体阴极上的析出电位较在固体钼上分别偏正0.86~1.23伏和0.66~1.13伏。这与钕和钇在液体阴极中的活度降低有关。由φ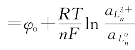 知,当
知,当![]() 时,较之固体钼阴极
时,较之固体钼阴极![]() 的情况下,φ要向正方向偏移。若阴极中稀土金属的浓度(活度)低至10-11(摩尔分数)数量级以下,可使φ偏正0.8伏以上(t=800℃),这在稀土离子开始析出的瞬间是完全可能达到的。
的情况下,φ要向正方向偏移。若阴极中稀土金属的浓度(活度)低至10-11(摩尔分数)数量级以下,可使φ偏正0.8伏以上(t=800℃),这在稀土离子开始析出的瞬间是完全可能达到的。
表1 钕和钇在钼和液体阴极上的析出电位(t=800℃,φcl-/cl2=0,XNdCl3=XNYCl3=1.75×10-2)
Table1 Deposition potentials of neodymium ion and yttrium ion on liquid cathodes at 800℃
误差按![]() 计算,范围为±0.02伏。
计算,范围为±0.02伏。
钕离子在各种液体阴极上析出电位依锑、铋、镓、饧、锌、铅、铟的顺序变负。在固、液体阴极上析出电位差Δφ的顺序为:锑>铋>镓>钖>锌>铅>铟。将此顺序和元素周期表对照发现:同一周期的金属做阴极时,Δφ从左向右递增(锑>钖>铟;铋>铅;镓>锌);同一族金属做阴极时,Δφ自上而下减小(锑>铋;镓>铟;钖>铅)。显然,这样的顺序和阴极金属的电负性规律基本一致[6]。也就是说,随着液体阴极金属电负性的增大,Δφ也变大。
钇离子在各种液体阴极上析出电位大小的顺序基本上与钕离子析出时一致,只有锌阴极例外,这可能和锌阴极的特殊极化过程有关。
在所研究的阴极上,钇离子的析出电位均较钕离子偏负,与纯氯化钇和氯化钕理论分解电位序相反。这是由于钇离子(离子半径0.88Å)的熔剂化作用较钕离子(离子半径0.99A)强所致。钕离子在钼和各种液体阴极析出电位差Δφ较钇离子在相应阴极析出电位差大,这与钕较钇和液体阴极金属电负性差值更大相一致[6]。
为了进一步研究钕和钇在液体阴极上析出电位较在固体钼阴极上向正偏移的原因,我们对钕在锑阴极,钇在钖和锌阴极上电沉积后的样品进行了X射线衍射分析,见图1,衍射图表明:钕和钇与液体阴极生成了多种金属间化合物。这有力地揭示了合金化作用是钕和钇在液体阴极上较在固体钼阴极上的析出电位向正方向偏移的重要原因。
图1 沉积在锑阴极上的钕和沉积在锡和锌阴极上的钇的X射线衍射图
Fig.1 X-ray diffraction diagram of neodymium deposited on Sb cathode and yttrium deposited on Sn and Zn cathodes(a)1—NdSb;2—Nd;3—Sb(b)1—YZn5;2—Y2 Zn17;3—Zn(c)1—Sn10 Y5;2—Sn10 Y11;3—Sn4 Y5;4—Sn
(二)YCl3-KCl.NaCl熔体中锌阴极的极化过程
YCl3-KCl·NaCl熔体中,锌阴极的伏-安曲线如图2所示,这在所研究的液态阴极极化过程中是特殊的。
图2中a区是锌阴极在熔体中腐蚀溶解的Zn2+放电,b区可能是钇离子在ZnO上析出,这与锂离子在锌阴极上析出相似;c区相应为钇离子在锌上析出。由d区可见,当电流密度>0.46安/厘米2时,碱金属开始析出。
图2 YCI3-KCI·NaCI熔体中锌阴极的极化曲线(XYC13=1.75×10-2)
Fig.2 Polarization curve of Zn cathode in YCI3-KCl·NaCl melt at 800℃
(三)温度对钕和钇析出电位的影响
不同温度下,钕在钼、铅和锑阴极,钇在钼和锡阴极上析出电位的实验结果列于表2,并用线性回归法给出了析出电位与温度(700~850℃)的关系式。
表2 不同温度下钕和钇在阴极上的析出电位(φcl/cl2=0,XNdCl3=XYCl3=1.75×10-2)
Table 2 Deposition potentials of neodymium and yttrium on the cathods at different temperatures
钕和钇在钼或液体阴极上的析出电位,都是随着温度升高向正向偏移,而温度对Δφ的影响较小。
(四)稀土氯化物浓度对钕和钇析出电位的影响
氯化钕和氯化钇浓度分别对钕在钼和锑阴极,钇在钼和锡阴极析出电位影响的实验结果列于表3,并给出了钕和钇在钼阴极上析出电位与它们氯化物浓度的关系式。
表3 NaCl3和YCl3浓度对钕和钇在阴极析出电位的影响(φcl-cl2=0,t=800℃)
Table 3 Influence of NdCl3 and YCl3 concentrations on deposition potentials of neodymiumion and yttrium ion on the cathods
NdCl3浓度对钕离子在钼阴极析出电位影响的实验关系式与从Nernst方程得出的φ=-2.86+0.213 lg x(t=800℃)较好地符合。外推法得到纯NdCl3(x=1)的分解电位(-2.90±0.02伏)与热力学计算值(-2.86伏)相近,而在锑阴极上呈现出较复杂的情形。
YCl3浓度对钇离子在钼和饧阴极析出电位影响,呈现和Nd Cl3浓度对钕离子在钼和锑阴极析出电位影响相类似的情况。
我们在实验中发现等摩尔KCl·NaCl体系较YCl3-KCl·NaCl(XYCl3=1.75×10-2)在锑和铋阴极上呈现出稍偏正的放电电位。为此,将YCl3-KCl·NaCl体系中锑和铋阴极极化后的样品进行了原子吸收光谱和发射光谱分析。结果表明,阴极中同时含有钾、钠和钇。这说明当XYCl3=1.75×10-2时,钾,钠和钇能在锑和铋阴极上共同析出,这在稀土中间合金的制备过程中是应当加以避免的。
四、小结
(1)测定了NdCl3-KCl·NaCl和YCl3-KCl·NaCl熔体、800℃下钕和钇在固体钼以及液体锑、铋、镓、铝、锡、锌、铅和铟阴极上的析出电位。钕和钇在上述液体阴极上析出电位均较在固体钼上明显偏正。其原因是钕和钇在液体阴极中的活度较固体钼上大大降低,以及合金化作用形成金属间化合物所致。在液、固体阴极上析出电位差Δφ随着钕和钇与阴极金属电负性差的增大而增大。
(2)钇在钼和上述各种液体阴极上析出电位较钕为负,这是钇离子的熔剂化作用较钕离子强造成的。钕在液、固体阴极上析出电位差Δφ较钇大,与钕和液体阴极金属电负性差较钇大是一致的。
(3)YCl3-KCl·NaCl熔体中,YCl3含量低时,锑和铋做阴极易发生钾、钠、钇共析。即锑和铋做为电沉积钇的阴极是不理想的。钇在锌阴极上的极化曲线形状比较复杂。
(4)研究了温度,NdCl3和YCl3浓度对钕及钇在钼、铅、锑和锡阴极析出电位的影响,给出了相应的关系式。
参加本工作的还有崔秀珍,杜富英和路连清同志。赵奇金同志现在有色金属研究总院工作。
参考文献
[1]Кокорин,М.Nилр.,Изв.ВУЗ,Цветная метаппургия,1969,(4),42.
[2]Голъгштейн,С.Л.идр.,Изв.ВУЗ.Цветнаяметаллургня,1976,(6),57.
[3]Буторов.В.П.идр.,Изв.ВУЗ.Цвтная металлургня,1971,(3),85.
[4]Баяяов.А.Л.,Изв.ВУЗ.Цвтнаяметаллургня,1969,(2),98.
[5]赵敏寿、唐定骧等,中国科学院长春应用化学研究所集刊,1983,(20),91.(https://www.xing528.com)
[6]尹敬执,申泮文编,基础无机化学,人民教育出版社,1980,52.
Electrochemical Behavior of Yttrium Ion in LiCl-KCl-NaCl Eutectic Melt[41]
S.Hikino,Gang Xie,K.Ema,Y.Ito
(Department of Nuclear Engineering,Faculty of Engineering,Kyoto University,Sakyo-ku,Kyoto 606,Japan)Zhao Min Shou
(Changchun Institute of Applied Chemistry,Academia Sinica,Changchun 130022 China)
Abstract:The electrochemical reduction of yttrium ion on a molybdenum electrode in a LiCl-KCl-NaCl eutectic melt at 723 K was found to be almost reversible and to proceed by a onestep three electron reaction.The diffusion coefficient D of the Y(Ⅲ)ion was measured to be(3.3±0.4)×10-6 cm2 s-1 by cyclic voltammetry,(5.0±0.9)×10-6 cm2 s-1 by the rotating disk electrode method,and(7.1±0.7)×10-6 cm2 s-1 by chronopotentiometry.The D values obtained by the latter two methods are in fairly good agreement with each other.The rather low D value obtained by cyclic voltammetry might be attributed to the fact that yttrium metal can dissolve slightly in the chloride melt.The standard potential of Y(Ⅲ)/Y(0)couple was determined to be(-3.174±0.006)V(v s.Cl2/Cl-)by open-circuit potentiometry,(-3.15±0.02)V(v s.Cl2/Cl-)by the rotating disk electrode method and(-3.16±0.02)V(v s.Cl2/Cl-)by chronopotentiometry.These three values are in good agreement with each other.Several types of Ni-Y intermetallic compounds were found to be formed on a nickel electrode.
Yttrium is widely used as an alloying element[1].The preparation of a yttrium containing master alloy,which is the dominant type used in practice,is mainly performed by electrolysis in molten chlorides[2].However,few papers have dealt with the electrochemical properties of Y(Ⅲ)ion in chloride melt[3-5].Thus,the present work has been conducted to elucidate the electrochemical behavior of yttrium ion in a 55LiCl-36KCl-9NaCl mole percent(m/o)eutectic at 723 K by cyclic voltammetry,the rotating disk electrode(RDE)method,chronopotentiometry and open-circuit potentiometry at molybdenum and nickel electrodes.The formation of several types of Ni-Y intermet-allic compounds is demonstrated by x-ray diffraction spectra.
Experimental
LiCl,KCl,and NaCl were all of reagent grade(Wako Chemical Co.,Ltd).The anhydrous YCl3 was obtained by slowly heating a mixture of YCl3·6H2 O and NH4 Cl under vacuum.Molybdenum or nickel wire of 1 mm diam and a molybdenum disk of 7 mm diam were used as the working electrode and RDE,respectively.To measure the electrode potential,a stabilized zirconia-air electrode was used as a quasi-reference electrode[6]for convenience,and the measured values are presented in the figures relative to the M+/M electrode potential.Here,M represents an alkali metal(Li and Na),and the potential of M+/M remains constant value,(-3.627±0.006)V v s.Cl2/Cl-,though the exact composition of M is not defined.A glassy-carbon rod of 5 mm diam was used as the counter electrode.The LiCl-KCl-NaCl eutectic melt was contained in a high purity alumina crucible(99.5%Al2O3,SSA-S Nippon Kagaku Togyo Co.,Ltd)and heated to be fused under dry argon atmosphere.After anhydrous YCl3 was added to the eutectic,dry HCl gas was passed through the eutectic for 30 min to eliminate any yttrium oxychloride that may exist in the anhydrous YCl3 or may be produced during the process of dissolving anhydrous YCl3.Then dry argon gas was bubbled through the electrolyte for 1 h to purge away residual HCl gas from the melt.All experiments were performed at 723 K.The experimental apparatus was almost the same as previously described[7].
Fig.1 Voltammogram:0.184 mol l-1 YCl3,Scan rate:100 mV/s
Results and Discussion
Electrochemical reduction of Y(Ⅲ)ion.-Figure 1 shows typical cyclic voltammograms obtained for electrochemical reduction of Y(Ⅲ)ion at molybdenum and nickel electrodes.A sharp reduction wave with a peak potential of 0.35 V(v s.M+/M)is observed at a molybdenum electrode;on the other hand,several reduction waves are observed at a nickel electrode.These cathodic peaks all have their corresponding anodic peaks.From the cyclic voltammograms with different scan rates at the Mo electrode,it is found that the cathodic peak potentials hardly change with scan rate.This means that the electrochemical reduction of Y(Ⅲ)ion is actually a reversible process at a molybdenum electrode.In the case where the product,yttrium metal,is hardly soluble,the following formula is preferred for calculating the number of electrons involved in the electrode reaction[8,9]
Fig.2 Peak cerrent density vs.square root of scan rate
Fig.3 Peak current density vs.concentration of YCl3
After correcting for ohmic drop,n,the number of electrons involved in the electrode reaction,can be estimated to be 2.8±0.4,which is smaller than but close to 3.Here,Ecp;and Ecp/2 are the cathodic peak potential and half peak potential,respectively.R,F,and T have their general significance.Thus,the electrochemical reduction of Y(Ⅲ)ion at a molybdenum electrode can be concluded to be a onestep three electron reaction:Y(Ⅲ)+3e=Y.As shown in Fig.2 and Fig.3,the plot of cathodic peak current density,ip,against the square root of scan rate is linear,and ip increases linearly with increasing YCl3 concentration.These results show that the reduction is diffusion controlled and the following equation is applicable[8]
Here,ip is the peak current density(m A cm-2),C,the bulk concentration of active species(mol l-1),V,scan rate(vs-1),D,diffusion coefficient(cm2 s-1).Figure 4 shows the linear dependence of normalized ip C-1 on the square root of the scan rate.From the slope of Fig.4,the diffusion coefficient of the Y(Ⅲ)ion is estimated to be(3.3±0.4)×10-6 cm2 s-1.
Fig.4 Normalized peak current density vs.square root of scan rate
Fig.5 lD~ED curve at rotating Mo disk electrode:0.184 mol l-1 YCl3,ratating velocity of disk:100 rpm
As described below,this value is rather low compared to the values obtained by other methods.One of the possible reasons is that yttrium metal can dissolve slightly in the chloride melt.In fact,if the equation for the reversible deposition of a soluble substance[13]
is used instead of Eq.(2),D can be estimated to be(6.1±0.8)×10-6 cm2 s-1.The situation is the same regarding the electron number,n.Considering the slight dissolution of yttrium metal,the true electron number n will be larger than the value calculated by Eq.(1).When D is calculated using a rotating disk electrode or chronopotentiome-try method,this slightly soluble nature of the yttrium metal does not affect the determination of D values as seen in the theoretical derivations of Eq.(4)and(9)[8,11].
RDE method.-The experiment was carried out at a rotating disk electrode with the electrode rotation velocity between 25 and 1000 rpm to determine the standard potential of Y(Ⅲ)/Y(0)and the diffusion coefficient of Y(Ⅲ).A typical disk currentdisk potential curve is shown in Fig.5.Only one plateau was observed,which corresponds to the reduction of Y(Ⅲ)ion at a molybdenum electrode.Figure 6 shows a linear relationship between the limiting current density iL and the square root of the rotation velocityω1/2.It means that the Levich equation(10)is applicable and the reduction is diffusion controlled
Here,in is the diffusion limiting current density(mA·cm-2),v,the kinematic viscosity(a value of 0.0148 cm2 s-1 for LiCl-KCl is used as an approximation),w,the angular velocity of the disk(rads-1).
Fig.6 Limiting current density vs.square root of rotating velocity
Fig.7 Chronopotentiogram at Mo electrode:0.368 mol l-1 YCl3,i=168.15 mA cm-2
From the slope of Fig.6,D is estimated to be(5.0±0.9)×10-6 cm2 s-1.
For the reversible deposition of an insoluble substance,the formula
can be derived[8].At one half the limiting current value
Here,Eo is the standard potential(V).From Fig.5,EiL/2 is(0.43±0.01)V v s.M+/M.Eo is thus estimated to be(0.48±0.01)V v s.M+/M,or(-3.15±0.02)V v s.Cl2/Cl-.
Chronopotentiometry.—A chronopotentiogram of Y(Ⅲ)ion in the melt at a molybdenum electrode is shown in Fig.7.Only one plateau appears,giving further proof for a one-step electrochemical reduction of Y(Ⅲ)ion.
For the reversible deposition of an insoluble substance,the following formula can be derived[8,11]
Here,Eo is the standard potential(V).When t=τ/4
Eo can thus be estimated to be(0.47±0.01)V v s.M+/M,or(-3.16±0.02)V v s.Cl2 Cl-from Fig.7.Straight lines for plots of it1/2 v s.i are obtained,as shown in Fig.8 on various concentrations of Y(Ⅲ).Figure 9 shows the linear relationship between iT1/2 and C.The line goes through the origin of coordinates which further verifies that the electrochemical reduction of Y(Ⅲ)ion is reversible and diffusion-controlled.These results imply that Sand equation[11]
is applicable.
Here,i is current density(m Acm-2),τ,transition time(s).From the slope of Fig.9,D can be calculated to be(7.1±0.7)×10-6 cm2 s-1.
Fig.8 Relation between iτ1/2 and i
Fig.9 Relation between iτ1/2 and C
Open-circuit potentiometry.-Pure yttrium deposits are formed on a molybdenum electrode by constant current electrolysis with cathodic current densities of 70—145 mAcm-2.The deposits are sufficiently stable to present the same characteristics as pure yttrium.A typical potential decay obtained after electrolysis is shown in Fig.10.A potential plateau remains stably at 0.437 V(vs.M+/M)for more than 5 min,then slowly shifts toward the positive potential and finally arrives at the rest potential of molybdenum in the melt.A plot of the plateau potential E against log C gives a linear relationship with a slope of(0.053±0.003)V decade-1,which is very close to the Nernst slope of 0.048 V decade-1 for a reversible transfer of three electrons at this temperature,as shown in Fig.11.A least squares calculation gives the molarity-scale standard potential Eo as(0.453±0.006)V vs.M+/M,or(-3.174±0.006)V vs.Cl2/Cl-.
Comparison between present results and the earlier work.-Y(Ⅲ)/Y(0)couple was studied in the 59LiCl-41KCl(m/o)eutectic earliest by Yang and Hudson[14],from whose data Plambeck[15]calculated the standard molarity-scale potential of this couple to be-2.859 V against the standard molar platinum electrode(S.M.P.E.).This value was obtained by extrapolating the data taken at temperatures higher than 723 K to 723 K.The standard molarity-scale potential of this couple in the 59 LiC1-41 KC1(m/o)eutectic was redetermined as-2.831 V(vs.S.M.P.E.)by Hoshino and Plambeck[4].Although the eutectic composition of the present study is different from those used in previous works,a comparison between the results of those works and the result of our study will give a better appreciation of the electrochemistry of yttrium and yield insights into the factors affecting the reduction reaction and the re-duction potentials.Such a comparison is summarized in Table 1,and the standard molarity-scale potentials given by the previous works are translated into potentials vs.Cl2/Cl-to make the comparison more clearly.Unfortunately,diffusion coefficient of Y(Ⅲ)ion was not given by those authors,it is impossible to make a direct compari-son.It can be seen from Table 1 that in all the cases,the reduction of Y(Ⅲ)is determined to be a one-step three elec-tron reaction without regard to the electrolyte used,and the standard molarity-scale potentials of the present study are very similar to those given by the previous authors,it seems that the sodium ion does not interfere with the reduction of Y(Ⅲ)under the experimental conditions.The difference in composition of the electrolyte used between present study and the previous works is not significant.
Fig.10 Open-circuit potential decay curve of Mo electrode after polarization at 145 mA cm-2,0.368 mol l-1 YCl3.
Table 1 A comparison between present results and the earlier works
Formation of Ni-Y alloy on nickel,disk electrode.—It is well known that nickel can form several types of interme-tallic compounds with yttrium[12].On the other hand,several reduction-oxidation waves are observed in the voltammogram obtained with a nickel electrode in Fig.1.In order to correlate these phenomena,electrodeposition of yt-trium on a Ni-disk electrode was carried out by constant current electrolysis in the melt between 723 and 873 K.Figure 12 shows a typical x-ray diffraction pattern for the deposit prepared under these conditions.The existence of NiY and Ni2 Y can be observed,which corresponds to the appearance of more than one reduction-oxidation wave in Fig.1.Depending on the electrolytic conditions(current density,temperature and so on)Ni2 Y3 can be formed also.Details of this electrochemical intermetallic compound formation reaction will be presented by the authors in due course.
Fig.11 E vs.log C
Fig.12 X-ray diffraction spectrum of Ni-y alloy
Conclusion
The results described above show that the electrochemi-cal reduction of Y(Ⅲ)ion is almost reversible and proceeds by a onestep three electron reaction on a molybdenum electrode in LiCl-KCl-NaCl eutectic at 723 K,i.e.Y(Ⅲ)+3e=Y(0).The diffusion coefficient of Y(Ⅲ)ion was determined to be(3.3±0.4)×10-6 cm2 s-1 by cyclic voltammetry,(5.0±0.9)×10-6 cm2 s-1 by a rotating disk electrode method and(7.1±0.7)×10-6 cm2 s-1 by chronopotentiometry.The value of D obtained by cyclic voltam-metry is different from those by the other two methods.One of the possible reasons is that yttrium metal can dissolve slightly in the chloride melt.The standard molarity-scale potential was estimated to be(-3.174±0.006)V(vs.Cl2 Cl-1)by open-circuit potentiometry,(-3.15±0.02)V(vs.Cl2/Cl-)by the rotating disk electrode method,and(-3.16±0.02)V(vs.Cl2/Cl-)by chronopotentiometry.These values are in good agreement with each other.Depending on electrolytic conditions,several types of Ni-Y alloy are formed at a nickel electrode,which was demonstrated by x-ray diffraction spectra.
Acknowledgment
This work was supported by a Grantin-Aid from the Japanese Ministry of Education,Science and Culture.
Manuscript submitted Oct.4,1991;revised manuscript received March 9,1992.Kyoto University assisted in meeting the publication costs of this article.
免责声明:以上内容源自网络,版权归原作者所有,如有侵犯您的原创版权请告知,我们将尽快删除相关内容。




